In the previous articles, we spoke about the capabilities of a vortex layer device in terms of grinding, mixing, and activating various substances. But the device can also be used to run and accelerate chemical reactions. One of such reactions is the reduction of sodium bisulfite in rongalite production.
What is rongalite and where is it used?
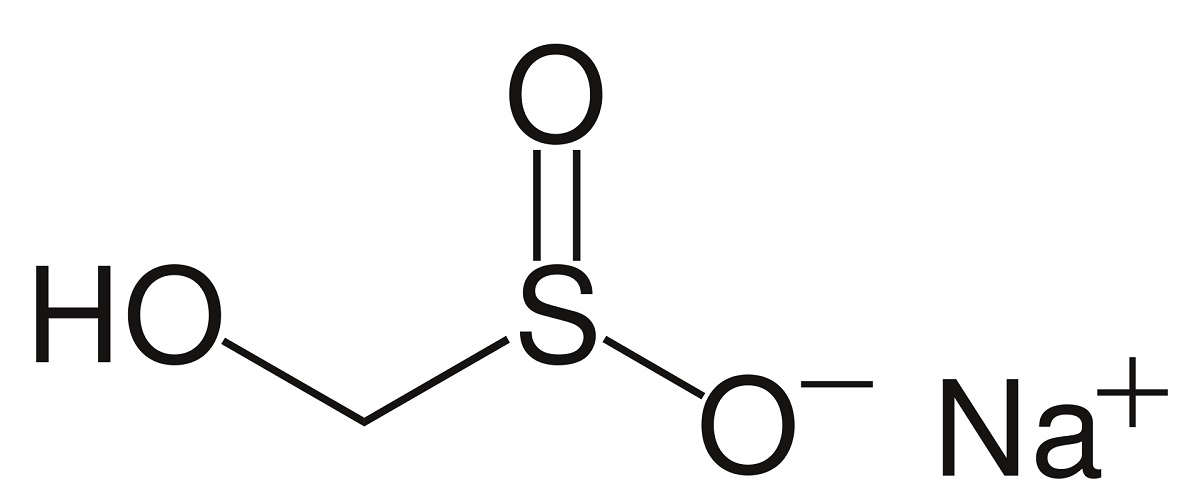
Rongalite is a chemical substance, the sodium salt of rongalitic acid, which does not exist in free form. Under normal conditions, rongalite is crystalline, white, and readily water-soluble. But it does not dissolve in benzene, ether, and ethanol. The specificity of industrial application of rongalite is determined by its good reducing properties.
Thus, rongalite is used:
- for bleaching various materials, including fabrics;
- as a reducing chemical agent, for example, in the composition of cosmetic products when removing the hair dyes;
- as an antioxidant in pharmaceutical products;
- in synthetic rubber production.
Rongalite production — baseline technologies
There are plenty of known methods for rongalite production, and all of them are associated with complex chemical transformations. These include:
- Interaction of formaldehyde with zinc and sulfur dioxide in an aqueous medium after which the reaction mixture is treated with zinc and sodium hydroxide.
- Hydrogenation of oxymethyl sulfonate in the presence of a catalyst (nickel) at the pressure of 120 atmospheres and the temperature of 50 degrees Celsius.
- Cathodic reduction of the zinc salt of oxymethylsulfinic acid with formation of zinc formaldehyde sulfoxylate which is then treated with sodium hydroxide.
All the mentioned methods for rongalite production are distinguished by a long duration of process and a low yield of target product.
Rongalite production by reduction of sodium bisulfite with zinc in the presence of formaldehyde appears to be more promising. It is followed by neutralization, filtration, evaporation, and crystallization. The yield of finished product with this technology can reach 80–90%. And the process can be accelerated if a vortex layer device is used as a reactor.
Vortex layer device as a chemical reactor
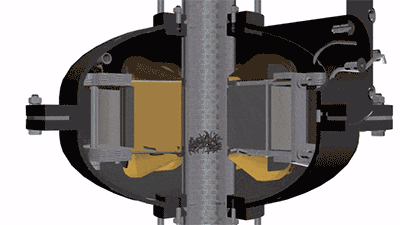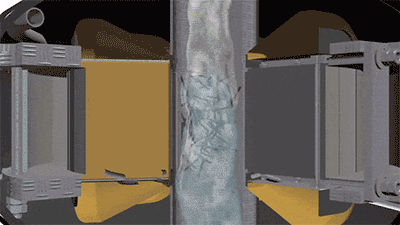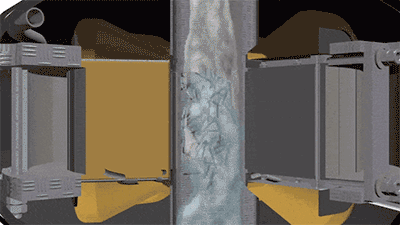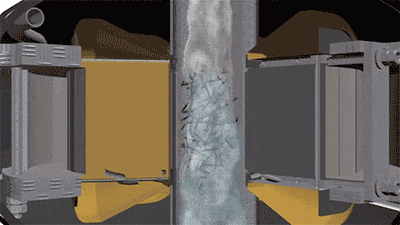 The operation of a vortex layer device is based on a rotating electromagnetic field which is created by the inductor winding. If an operating chamber made of a non-magnetic material is placed into this field, and the chamber is filled with needle-shaped ferromagnetic particles, then the particles begin to move along complex trajectories. It will be facilitated by constant collisions of particles with one another, with the operating chamber walls, and with processed substance. At the same time, there arise physical and chemical processes in the operating chamber that can intensify the course of chemical reactions (magnetostriction of ferromagnetic particles, electrolysis, acoustic vibrations, intensive mixing, etc.). As a result of their cumulative influence, the chemical reactions that last for tens of minutes and several hours under normal conditions proceed much faster — for several seconds and tens of seconds. For example, the first stage of rongalite production involves feeding the water and the zinc dust into the reactor. In order to obtain zinc pulp, it is recommended that mixing be carried out for 15 minutes. In a vortex layer device, a homogeneous zinc pulp can be obtained in just a few seconds. The reduction reaction of sodium bisulfite added to the reactor after obtaining the zinc pulp proceeds much faster as well.
The operation of a vortex layer device is based on a rotating electromagnetic field which is created by the inductor winding. If an operating chamber made of a non-magnetic material is placed into this field, and the chamber is filled with needle-shaped ferromagnetic particles, then the particles begin to move along complex trajectories. It will be facilitated by constant collisions of particles with one another, with the operating chamber walls, and with processed substance. At the same time, there arise physical and chemical processes in the operating chamber that can intensify the course of chemical reactions (magnetostriction of ferromagnetic particles, electrolysis, acoustic vibrations, intensive mixing, etc.). As a result of their cumulative influence, the chemical reactions that last for tens of minutes and several hours under normal conditions proceed much faster — for several seconds and tens of seconds. For example, the first stage of rongalite production involves feeding the water and the zinc dust into the reactor. In order to obtain zinc pulp, it is recommended that mixing be carried out for 15 minutes. In a vortex layer device, a homogeneous zinc pulp can be obtained in just a few seconds. The reduction reaction of sodium bisulfite added to the reactor after obtaining the zinc pulp proceeds much faster as well.
Thus, the use of vortex layer devices as chemical reactors allows intensifying the processes of rongalite production. Moreover, the device has compact dimensions, can be used in a laboratory environment, and does not consume much power (not more than 4.5–10 kW depending on the model).