Produced water from oil and gas exploration and production. Man made bore holes cause many billions of cubic meters of water pouring out from the depths of the Earth and poison its surface and all living creatures in waterways. These include co-produced water in oil and gas production fields. The content of oil and gas can be only 15-20% the rest is water with dissolved oil, metals and gases. In some areas, it is practiced to inject this water back into the wells.
Millions of mines continuously pump out mine water and dump it without purification into rivers, many of which are already dead.
The purification of these waters are imperfect, and more often than not are non-existent.
There is another group of waters from boreholes that are drilled prospecting for oil, coal and other minerals or in search of water.
Most of these wells are rendered unproductive and are abandoned. But almost always they were drilled through water layers, and the strata water gush, flooding the surrounding areas. Their composition is not often determined but they always contain dissolved compounds of elements that are harmful to flora and fauna, but may prove useful to humans (Table 54).
Table 54
The content of elements in the waters of the Mesozoic deposits
|
|
Content, mg / l |
|||||||
|
V |
Со |
Ni |
Сu |
Zn |
Мо |
Рb |
Аq |
|
|
|
trace- 129 |
trace- 39 |
trace- 130 |
0,5-250 |
trace- 4200 |
trace 16,6 |
trace- 12,2 |
No information |
|
average-9,8 |
average 7,1 |
average 34,1 |
average 23,6 |
average 731 |
average 4,5 |
average 2,1 |
||
|
|
trace–16 |
trace–39 |
trace– 112 |
0,6-80 |
trace–2240 |
trace– 19,8 |
trace– 10,9 |
No information |
|
1,6 |
3,3 |
12,5 |
7,5 |
226 |
2,8 |
2,5 |
||
|
trace- 176 |
trace– 4,5 |
trace–264 |
0,5-72,6 |
trace–1660 |
trace- -23 |
trace– 92,5 |
No information |
|
|
15,5 |
4,4 |
35,4 |
6,7 |
176 |
3,8 |
4,9 |
||
|
No info |
trace–20 |
No info |
No info |
trace– 100 |
trace-100 |
trace– 20 |
trace- -100 |
|
Big data has been collected on the composition of co-produced water. All of them without exception contain precious and nonferrous metals. Very often there is a presence of precious metals.
The content shown in Table 54 is quite impressive. Also, with depth, in particular, and with an increase in temperature, the content of elements in water increases by 2 or even 25 times.
Often, water has a strong head and gush, reaching 6,000 m3 / day, and the content of metals reaches 200 g / l. Such concentration of harmful elements, sharply endangered flora and fauna and exaggerates environmental degradation in the region.
The data showed that the content of metals in the Mesozoic deposits is greater than in the world’s ocean’s in tens or even hundreds of times
There is a significant percentage of alkali and alkaline-earth metals, and the content of sulfate ions S042- and Cl is great too, but the content of valuable components is so great that many researchers have considered their production [163-166].
However, traditional methods of extracting metals in relatively low concentrations need a high budget for roads, buildings, heating plants, fuel, and so on.
The AVS changes this situation.. These machines, can easily cope with 6,000 m3 / day flow rate, not requiring buildings and large settling ponds, they also have minimal energy consumption. Therefore, they have a large economic and ecological benefit by eliminating dangerous elements for man and nature.
As an example, let’s consider the neutralization and utilization of produced water. Their special feature is a great silver content. The technological flow sheet is shown in Fig. 150.
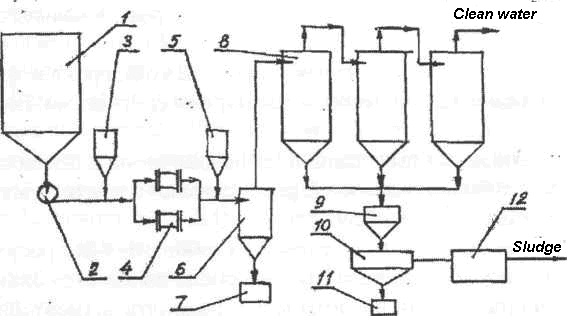
Fig. 150. Flow sheet of neutralization and utilization of produced water from drill holes. 1 – Stabilization tank 2 – Pump; 3, 5 – Containers for additives; 6 – Intermediate tank; 7.9 – Collection tanks; 8-active sedimentation tank; 10 – Hydro separator; 11 – Collector of metals; 12 – Accumulator of compounds of metals
The water from the Stabilization tank 1 is fed by pump 2 with additives from tanks 3 into the AVS 4, with a coagulant from tank 5 through intermediate tank 6 into sedimentation tanks 8. The purified water is discharged into a river, pond or used for irrigation
This technology reduces silver compounds by the iron needles and by electrolysis in the processing chamber of the AVS [1]. At the same time, the metal compounds are isolated from the solution in the form of sludge. The bulk of the silver, being heavier is separated from the slurry and accumulated in the intermediate tank 6, and collected in the collection tank 7. Metal compounds are first collected in a collection tank 9 are separated into the active sedimentation tank 8. The remaining part of the silver and the platinum group metals are separated in the separator 10. Sludge is collected in a container 12 with a removable container, packed and sent for processing
The flow sheet in Fig. 150 differs a little from sludge and wastewater treatment The difference is a possible production of metals: silver, gold or platinum.
It should be understood that the amount of sludge is quite noticeable. So, from the well with a flow rate of 1000 m3 / day, its yield taking into account the ions contained in water S042- of chlorine, sodium, calcium and other with be about 3 tons per day, In terms of thickened sludge – 6-7 tons / day, in the year 2000-2100 tons
In Astrakhan region, there is a significant deposit of bromine and iodine form the water coming from several wells. A representation of the composition of the waters of some of them is given in Table 55.
Composition of water from wells
|
Groups of deposits |
|||||||
|
Items |
I |
II |
|||||
|
Items |
A -1 | А – 2 | А – 3 | А-4 | B – 1 | B – 2 | B – 3 |
|
Depth, m |
100 |
280 |
870 |
Surface |
3350 |
3600 |
330 |
|
Mineralization, g / l |
15 |
23 |
175 |
100 |
121 |
328 |
290 |
|
Bicarbonate, mg / g |
134 |
100 |
49 |
60 |
275 |
275 |
61 |
|
Calcium, mg / l |
2180 |
1218 |
1523 |
1200 |
11423 |
26650 |
11825 |
|
Magnesium, mg / l |
– |
– |
– |
– |
385 |
3405 |
1945 |
|
Iodine, mg / l |
22 |
26 |
23 |
29 |
121 |
127 |
42 |
|
Bromine, mg / l |
14 |
45 |
100 |
204 |
464 |
1438 |
864 |
|
Working rate, m3 / day per group |
500 m3 / day |
to 1 000 m3 / day with lower aeration |
|||||
|
Iodine reserves, t |
21500 |
25100 |
28800 |
35600 |
85800 |
154400 |
145800 |
The table does not reflect the content of heavy, rare and rare-earth metals found in the produced waters in this region.
It is noticed that with more depth comes more iodine, bromine and other elements. The presented data reflect the reserves, which are contoured, but in fact, the reserves are many times greater.
The existing technologies for iodine isolation are based on chemical reactions, which require large tanks, acids and other reagents, with a problem of dumping the process waste.
As is well known, iodine is present in aqueous solutions of alkalis and carbonates in the form of NaJ and NaJO3. The salts of iodine acid are stable and are strong oxidizing agents. Salts HJO3 (iodates) in the presence of acid can emit elementary iodine, but with interaction with iodides. The resulting iodine is absorbed by the active carbon, which is treated with alkali, for example, NaOH. A solution of NaJ + NaJO3 is formed. From this solution, under the action of sulfuric acid, metal iodine is isolated (in the presence of elemental chlorine) by the reaction [168, 169]:
NaJO3+5NaJ+3H2SO4=NaSO4+H2O
Obtaining pure iodine with the AVS is much simpler and doesn’t use acids and chlorine. Table 55 does not include metals, but they can also be isolated (Figure 151.).
The technology is based on obtaining practically insoluble iodine compounds, which can be carried out only in the AVS. The additive for isolation of iodine from solutions (brine), returns to the head of the process. Iodine is purified by distillation. The compounds of rare and heavy metals are isolated according to the technology developed for sludge or wastewater treatment. The settler should select only iodine, whereas bromine passes almost unhindered
The recycling process is continuous. The water from the tank 1 enters the AVS 3. In front of the machine 3, an iodine precipitator is added from the additive tank 2. A solid fraction containing practically only the iodine compound is separated in the intermediate tank 4, and settled in the active sedimentation tank 6. The heavy fraction accumulates and rapidly compacts in the collector (sludge receiving tank) 13, The dryer 14 dries the mass and transfers it to a distiller 15 in which the iodine evaporates, gets trapped, separated, packaged and sent to the customer. The remainder is diluted with water or acid and returned to the process head in front of the AVS 3.
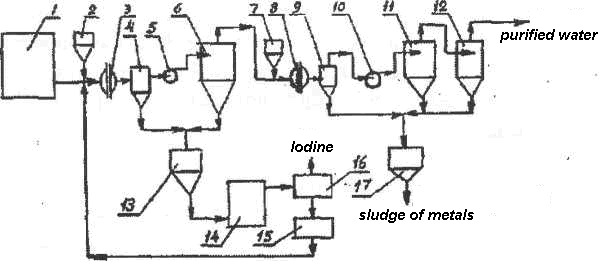
Fig. 151. Flow sheet of utilization of natural brine (with iodine): 1 – Tank for brine; 2,7 – Tanks for additives; 3, 8 – AVS; 4, 5 – Intermediate tank; 5, 10- Pump; 6, 11,12 – Hydro cyclones; 13, 17 – Sludge absorber; 14 – Dryer; 15 – Waste Collection tank; 16 – Distiller
A solution of metals from the sedimentation tank 6 enters the machine 8 with an additive from the tank 7 and passes the path similar to wastewater treatment and separation of heavy metals that concentrate in the form of a slurry in the sludge receiving tank 17
The result of processing co-produced water from drill holes are: iodine, metal compounds and water containing alkali metals and bromine. Bromine can be isolated according to the same scheme, with organic solvents or using the techniques described in [170].
A day’s yield from the wells of A group -could be 12.5 kg and from wells of B group -97 kg (according to the data in Table 56), provided that the same processing rate is maintained. Accordingly, up to 35 tons of iodine will be received in a year. The amount of iodine produced can fluctuate depending on the situation and on the yield.
To ensure the processing of such a volume of co-produced water will require from 1 to 6 AVS machines to simultaneously obtain, at least 80-90 tons of other metals compounds. The area of brine processing of boreholes will look as follows (Table 56).
Table 56
The main indicators of brine processing from boreholes (brine)
|
Indicators |
Units |
Data |
Notes |
| Performance:
– by iodine – by weight. metals |
t/year t/year |
30-32 80-90 |
|
| Power Consumption |
kW |
60 |
active 6 units on one frame |
| Personnel |
people |
5 |
for 1 shift |
Mine waters
Recently, the issue of neutralization, purification and utilization of mine waters became especially topical in connection with the mass closure of unprofitable mines of the country’s basins.
To date, there is practically no reliable data on deep purification of mine waters. The proposed methods of ion exchange chromatography, reverse osmosis, electrolysis and some other methods can not be considered acceptable, due to their low productivity, high energy costs, expensive chemicals, equipment complexity, etc.
Shaft waters vary in composition (Table 57), which makes it difficult to create any unified technology.
Reagent neutralization of water has become very widespread, and therefore there is no need to conduct a review of the methods. It should only be noted that natural waters with such high mineralization and hardness are difficult to process to obtain a composition suitable for further use in fish farms.
Therefore, only partial purification is considered.
Traditional methods will require large ponds – sedimentation tanks, a complex mixer system, significant production areas and large costs for equipment, energy and operation without any not promise for successful cleaning. In addition, it is also necessary to neutralize a large amount of iron and other metals, including heavy metals. Apparently, this is what led to virtually nonexistent cleaning systems of mine water.
A non-traditional system for the purification of natural waters with the AVS can not guarantee the complete elimination of mineralization, but it can reduce it due to almost complete precipitation of calcium and magnesium compounds, and also bring hardness to the level corresponding to the MPC norms, and simultaneously reduce the iron content and other metals, including heavy metals, to the MPC level.
It is not completely possible to eliminate the mineralization as it originally is sulfates and chlorides of alkali metals that are poorly soluble or insoluble in water. They can be removed only by distillation, by ion exchange chromatography or by various types of electrolysis, which were mentioned earlier.
However, the proposed method allows reducing the costs of reducing hardness many times by removing heavy metals, significantly speeding up the separation of liquid and solid phases, reducing costs for buildings and working area and reducing the consumption of reagents.
In the event that mine water contains predominantly carbonate and non-carbonate hardness, for example, water from wells No. 48 and 52, it can be brought to a composition that meets the requirements of hatcheries.
Table 57
Compositions of waters discharged from mines
|
Location of output water |
color |
рН |
Nа +К, mg/l |
Hardness, mg- эeq / l |
Са, mg/l |
Mq, mg/l |
Chlorides, mg / l |
Sulphates, mg / l |
Total Fe mg / l |
Ionammonium, mg / l |
Mineralization, mg / l |
| The mine, February 2001. | brown | 6,8 | 104 | 32,3 | 275,2 | 233,5 | 602,7 | 2343 | 14,92 | 0,01 | 15400 |
| Mine, 05.12.2000
Discharge of water |
yellow | 6,98 | 887,8 | 39 | 248 | 324,5 | 460,8 | 2728 | 21,0 | 2,00 | 16000 |
| Discharge of water in the river. Ayuta from the Nesvetaevsky seams | No info | 6,6 | 880 | 57 | 530 | 370 | 410 | 3600 | 12,1 | No info | 15800 |
| According to the letter “Rostovgiproshaht” | No info | 5,8 | 244 | 250 | 1600 | 2074 | 319 | 11850 | 330 | No info | 19000 |
| Mine- 8, borehole № 48 | No info | No info. | 569 | 14 | 265 | 10 | 693 | 293 | No info | есть | No info |
| Borehoole № 52 | No info | No info. | 552 | 21,6 | 405 | 17 | 473 | 257 | No info | есть | No info |
| MPC for public water bodies | Света.
прозрач ный |
6,5-8,5 | – | 7-10 | – | – | 350 | 500 | 0,3 | – | 1000 |
It should be noted that widely used soda or sodium hydroxide, while reducing the hardness of water, simultaneously increases mineralization. Therefore, they should be applied with great circumspection.
Neutralization of mine waters was based on known reactions:
CO2Ca(HCO3+Ca(OH)2=CaCO32CaCO3+H2O2H2O
Mg(HCO3)2MgCO3+Ca(OH)2=CaCO3+MgCO3Mg(OH)2+H2O
CaCl2CaSO4+Na2CO3=CaCO2+2NaClNa2SO4
Carbonate and noncarbonate hardness decrease. With the introduction of soda, mineralization can even grow.
Additives of caustic sodium (NaOH) were also tested.
Its influence on the composition change is described by the equations:
Ca(HCO3)2Mg(HCO3)2+2NaOH=CaCO3Mg(OH)2+NaCO3+H2O+1CO2
CO2+NaOHNa2CO3
CaSO$CaCl2+NaCO3=Na2SO42NaCl+CaCO2
MgSO4MgCl2+2NaOH=Na2SO42NaCl+Mg(OH)2
Hardness is quickly reduced, but mineralization is increased due to the formation of sulphate and sodium chloride. Simultaneously, the iron content is reduced to 0.5 mg / l. The disadvantage of the method is an increase in the pH of the medium – up to 10-11. But with this factor you can fight.
The use of known coagulants, polyacrylamide and aluminum sulphate, is likely to be ineffective, since at high pH, for example, aluminum sulfate does not form a sorption-active hydroxide.
Apparently, iron chloride should be used to accelerate precipitation. But at the same time, it also increases mineralization significantly.
Obviously, it is necessary to search for such additives that would be as effective as Na0H and Na2CO3, but would not increase the mineralization
The compounds of aluminum, NH4OH and (NH4) 2CO3 were tested. In addition, great prospects open with various combinations of additives. It is known that the solid particles, regardless of size that formed in the processing chamber of the AVS settle much faster than those formed in reactors with agitators. In addition, when filtered through a new sand filter, only the film type of filtration appears. These circumstances are of major technological importance, as they eliminate the traditional settling ponds.
The water, presented in Table 57, flows from mines or wells in quantities exceeding 1000 m3 / h. Such flow rate influences the kinetic factors which can shift these or other reactions often in an undesirable way, for example, a poor mixing of water with additives. As a result, the planned outcome is not achieved. The turbulent movements of water prevent the complete separation of phases and separation of sediment.
Therefore, the machine that is able to overcome these difficulties acquires a special significance.
Active settling tanks, which are a modification of hydrocyclones can be very effective.
No less difficult is the task of utilization of sludge, which accumulates in large quantities. Sludge is a mixture of magnesium hydroxides, calcium carbonates and magnesium with a small amount of sulfates and chlorides.
Rotating magnetic fields clearly affect the elemental composition of water, the physical properties of the sediment, as well as the effect of additives on mine water from the Glubokaya mine. The results are shown in Table 58 and in Fig. 152.
Table 58.
Change in the composition of mine water from the mine with the AVS treatment
| № | Additives, ml / l | pH | Total hardness | Suspended substances | Dry residue | |||||||
| CaO | NaOH | Na2COP3 | NH2OH | (NH4)2CO2 | mg-equi | Lower % | mg/l | Lower % | mg/l | Lower % | ||
| 1 | initial | 6 | 6.0 | 31 | – | 140 | – | 5921 | – | |||
| 2 | 16 | – | – | – | – | 6,5 | 15.0 | -52 | 43 | -70 | 5017 | -15 |
| 3 | – | 16 | – | – | – | 10 | 0.7 | -98 | 128 | -8.5 | 7255 | High
+22 |
| 4 | – | – | – | 20 | – | 10 | 16.2 | -51.6 | 79 | -43.5 | 4861 | -18 |
| 5 | – | – | – | 10 | 7.0 | 16.0 | -52.2 | 159 | +13 | 5018 | -15.2 | |
| 6 | 16 | 6 | – | – | – | 8.9 | 7.4 | -76 | 83 | -41 | 5124 | -13 |
| 7 | 16 | – | 5 | – | – | 8 | 10.3 | -67.0 | 246 | +75 | 4984 | -15.7 |
| 8 | 16 | – | – | 20 | – | 11 | 10.8 | -65 | 38 | -73 | 4558 | -33 |
Ferric chloride was used as a coagulant,. The additives were introduced as 10% solutions and changed depending on the task.. The water-lime slurry contained 5% CaO.
According to the tests performed at the mine, it was found that the content of Ca can drop from 275.2 to 4-8 mg / l, Mq from 233.5 to 4.88-5.6 mg / l. Apparently these components determine water hardness and this sharp change in their content reduces the hardness.
Considering the effect of additives shows that one additive doesn’t always lead to a desired result. More effective is an introduction of two or more additives. This relates to hardness. At the same time, the mineralization changes and not always in the required direction.
The testing (but not on the actual water composition) was influenced by the turbidity of water after treatment. The main part of sediment (95-98%) settled in the first 5-10 minutes. Then, after settling for 5-10 hours, the water is completely clarifie.
To eliminate this factor, the water was filtered through a layer of sand 5-6 cm thick; the increase in the thickness of the sand layer had no significant effect on the purity. As was shown above, the filtration significantly. improved the indicators on suspended substances and on mineralization.
Proposed for the first time ammonium compounds proved very useful in a number of cases in relation to mineralization, but the total hardness was not always in the range 7-8. These additives can be most effective when used with other additives that reduce hardness.
Experiments showed that neutralization and purification of mine waters within certain limits is possible and can be effective on an industrial scale.
However, the purification technology can not be unified and requires its development for specific waters, depending on the composition and quantity.
If the water has a N3 + K-sulfate-chloride mineralization, then its hardness will be reduced by purification from calcium, magnesium and iron. Hardness can be reduced to the maximum permissible concentration limit. Also, the content of iron and heavy metals is reduced to the MPC norms.
If the water has a magnesium-calcium mineralization, then the water can be purified to the purity permissible to the norms for fish-breeding reservoirs.
Two types of technological lines with implementation of the AVS are proposed:
One technology for mine waters clarified by rapid and complete precipitation and separation of the solid phase (Figure 153). After treatment the water is discharged into the pond.
Second technology for waters that retains a slight turbidity, the processing line must be equipped with an additional filter, for example, a sand filter or a floating-load filter (Figure 154).
The sediment formed during water purification can be used as a binder for briquetting coal coal or in the production of building materials where lime is required. Sludge application areas can be expanded with appropriate marketing.
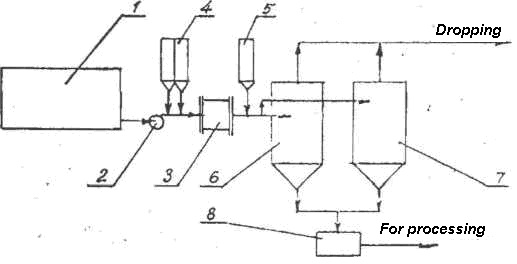
Fig. 153. Flow sheet of neutralization and cleaning of mine waters: 1 – Receiving tank; 2 – Pump; 3 – AVS; 4, 5 – Tanks for additives; 6.7 – Active sedimentation tanks; 8 – Sludge collection tank
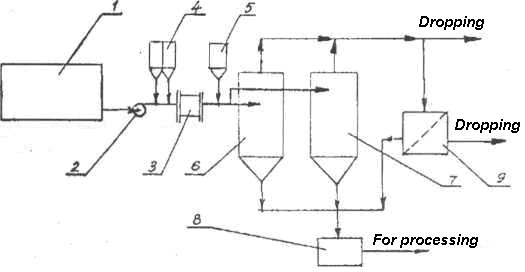
Fig. 154. Flow sheet of neutralization of mine waters with filtration: 1-8 – See Fig. 153; 9 – Filter
Thus, mine water and water from boreholes can be neutralized completely or partially, which depends on their initial composition.

