Titanium dioxide is a water-insoluble white powder, basically, a white dye. It is a very interesting substance, first of all, due to its wide application. Therefore, titanium dioxide production is an important component of the global industry.
Titanium dioxide production in the world
In the mid-2000s, 4.2 million tons of titanium dioxide were used worldwide. The largest producers of this chemical are the USA and China. Furthermore, large market shares are taken up by the UK, Japan, and Germany.
Titanium dioxide production uses titanium-containing ores:
- rutiles (titanium dioxide content — 93–96%);
- ilmenites (44–70%);
- leucoxenes (up to 90%).
The largest deposits of titanium ores are located in the USA, India, Australia, Brazil, South Africa, and Kenya.
Fields of titanium dioxide application
As we have mentioned before, titanium dioxide is widely applied in the industrial sector. Its largest users include:
- paint and varnish industry (59% of overall usage). The average proportion of titanium dioxide pigment in paints is 25%;
- plastics production (20%);
- paper production (13%). Titanium dioxide is used as a pigment and gradually replaces kaolin.
The production of natural rubber, artificial fibers, cosmetics, linoleum, plasters, and cement mortars accounts for a relatively small fraction of the overall titanium dioxide use.
Titanium dioxide production using ilmenite
Let us discuss titanium dioxide production using ilmenite. It can be conveniently divided into several stages:
- decomposition of ilmenite using sulfuric acid;
- separation into titanium sulfate solution and insoluble precipitate — iron sulfate;
- filtration, evaporation, calcination of titanium sulfate precipitate;
- reduction to obtain the finished product — titanium dioxide.
First, ilmenite should be prepared to react with sulfuric acid. For that purpose, it is dried to residual moisture content of not more than 1%; next, it is ground by means of energy-inefficient ball mills which consume tens and hundreds of kilowatts of power. After pulverization, the dispersion ability of titanium concentrate particles should be no greater than 0.056 mm. A small proportion of larger particles is allowed: it makes only 0.1% for a continuous process and 2–5% for a batch process.
The next stage involves feeding the almost powdery concentrate and the concentrated sulfuric acid into special reactors. That’s where ilmenite decomposes at the temperature of 200 °C.
The main components of ilmenite — TiO2, FeO, and Fe2O3 — react with sulfuric acid. As a result, TiOSO4, FeSO4, Fe2(SO4)3, and water are formed, and heat is released. However, the generated heat is still not sufficient to maintain the temperature at 200 °C; thus, the process requires additional energy consumption for heating.
Complete decomposition of ground ilmenite requires a high consumption of sulfuric acid. After decomposition, a sulfate alloy is obtained and takes from one to three hours to mature. After maturing and cooling down to 70 °C, the sulfate alloy in the same reactor is leached with slightly acidified water which results in conversion of titanium sulfate into a solution. Leaching takes several hours.
Then, ferric iron is reduced to ferrous iron by means of cast iron and iron filings; mechanical impurities are removed from the titanium sulfate solution; the solutions are crystallized and centrifuged in order to remove iron vitriol residues. After that, vacuum evaporation and calcination are carried out. After cooling, the obtained pigment is ground, packed in bags, and sent to the end user.
Let us summarize the disadvantages of this approach to titanium dioxide production using sulfuric acid:
- multistage nature and complexity of processes;
- high energy consumption;
- excessive sulfuric acid consumption;
- wastes generated in large quantities with some of them posing hazards (diluted hydrolytic sulfuric acid and iron vitriol);
- part of titanium raw stuff remains unprocessed.
Prospects of vortex layer devices in titanium dioxide production using ilmenite
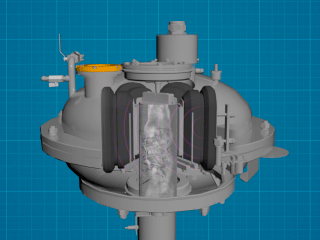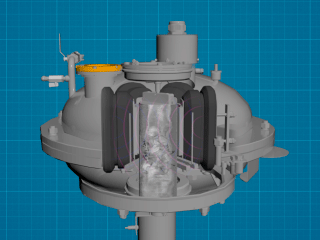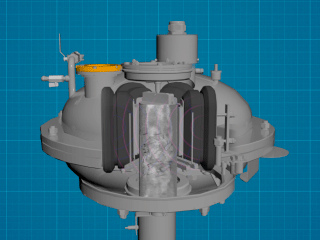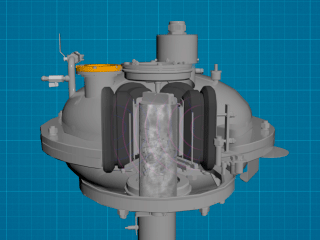 The listed disadvantages make it relevant to search for ways of enhancing the efficiency of titanium dioxide production. In this regard, we propose to discuss the possibility of introducing this kind of vortex layer devices (AVS) in technological lines.
The listed disadvantages make it relevant to search for ways of enhancing the efficiency of titanium dioxide production. In this regard, we propose to discuss the possibility of introducing this kind of vortex layer devices (AVS) in technological lines.
A vortex layer device is versatile equipment that can simultaneously grind, mix, activate, and accelerate chemical reactions. How did this versatility become possible? The answer can be found by analyzing the device design. A vortex layer device consists of a rotating electromagnetic field inductor, an operating chamber made of non-magnetic material placed inside the inductor, and ferromagnetic particles in an amount from several dozen to several hundred pieces. The amount and the geometric dimensions ratio of ferromagnetic particles depend on the type of technological process and may be different for each technological process.
After voltage is applied to the inductor winding, a rotating electromagnetic field is induced in the operating chamber, and as affected by this field, the ferromagnetic particles start moving and colliding with one another and with the operating chamber walls. As a result, the trajectory of each particle becomes complex, and a set of these trajectories forms a vortex layer. In this vortex layer, there arise a number of processes and phenomena that have a favorable effect on processed substances. These include:
- electromagnetic field effect;
- impact effects of ferromagnetic particles;
- high local pressures;
- ultrasonic vibrations;
- cavitation (in a liquid medium), etc.
As a result, the substances that enter AVS operating chamber are ground, mixed, and take on new properties. And chemical reactions are accelerated a ten- and hundredfold. The use of AVS appears to be reasonable based on the need to grind ilmenite concentrate, its chemical interaction with sulfuric acid, and the long duration of titanium dioxide production.
Titanium dioxide production using AVS — Experiment results
Two samples of ilmenite concentrate weighing 150 grams each were taken for the experiment. These samples were ground in the operating chamber of AVS-100 vortex layer device for 40 and 60 seconds respectively. After processing, both samples were forced through a sieve. The results are shown in Table 1.
Table 1 – Results of ilmenite concentrate grinding in AVS-100 vortex layer device
| Initial sample, mm | Sieve (cell), mm | Sample No.1 (40 s processing), % remainder on the sieve | Sample No.2 (60 s processing), % remainder on the sieve |
| 4.4 | 0.2 | 0.1 | 0.0 |
| 30.5 | 0.1 | 0.4 | 0.1 |
| 63.6 | 0.05 | 1.9 | 0.4 |
| 1.5 | Less than 0.05 | 97.6 | 99.5 |
As shown by obtained data, only forty seconds of processing is enough for efficient grinding of the sample.
After grinding, the samples were decomposed in sulfuric acid. Decomposition took a few seconds of processing. After that, they were diluted with water to the required concentration without removing the samples from the device operating chamber.
Advantages of vortex layer devices in titanium dioxide production
Applying AVS in the process of titanium dioxide production has the following advantages:
- Combination of several processes that can be carried out in the operating chamber of AVS: ilmenite concentrate grinding, decomposition with sulfuric acid, dilution with water. It means that AVS replaces mills and reactors which helps in reducing the size of the technological line and the floor space it occupies.
- Acceleration of the titanium dioxide obtaining process due to intensifying factors in the device operating chamber. The reaction of ilmenite decomposition with sulfuric acid proceeds in a matter of seconds.
- Great sulfuric acid savings due to a faster and more complete course of chemical reactions in the device operating chamber.
- Electricity savings. Compared to ball mills, AVS does not consume much power (4.5–9.5 kW depending on the model).
- AVS requires no special pedestals for installation and is easily integrated into existing technological lines instead of a mill or reactors.
In order to get advice from our technical professionals with regard to introducing AVS in technological lines for titanium dioxide production, please use some of the contact details contained in the appropriate website section.